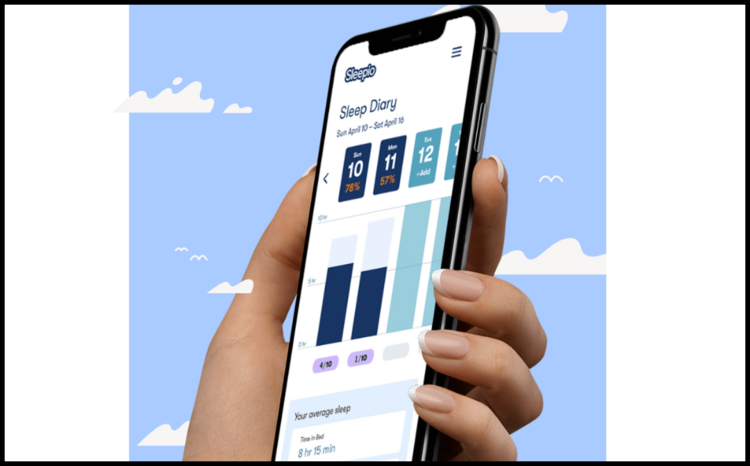
Insomnia app unavailable for most of NHS despite NICE approval
Big Health says that its app to treat insomnia is unavailable to most NHS patients despite being recommended by NICE
Read More